Pipeline
Our pipeline includes clinical development stage candidates for the treatment of recurrent genital herpes, chronic hepatitis delta virus (HDV) infection and chronic hepatitis B virus (HBV) infection. Our research programs focus on the discovery of additional novel antiviral small molecules for the treatment of serious viral diseases.

*Gilead contributed program; ** Assembly and Gilead combined program
ABI-5366 and ABI-1179
ABI-5366 and ABI-1179 are HSV helicase-primase inhibitors in clinical development for the treatment of recurrent genital herpes. These candidates target the helicase-primase complex, an essential viral enzyme complex that is conserved across both HSV-1 and HSV-2 and has no host equivalent. Inhibition of the helicase-primase complex is a clinically validated mechanism that has shown the potential for superior efficacy to nucleoside analogs in short-duration clinical studies in individuals with recurrent genital herpes. Both ABI-5366 and ABI-1179 are in development as long-acting agents for recurrent genital herpes targeting once-weekly or longer oral dosing intervals.
ABI-5366 has completed the Phase 1a portion of a Phase 1a/b study. In interim data released from the ongoing phase 1b portion of this study in individuals with recurrent genital herpes, a weekly oral dose of 350 mg of ABI-5366 showed statistically significant reductions in shedding rate and genital lesion rate compared to placebo over 29 days. The observed pharmacokinetic profile continues to support once-weekly and potentially once-monthly oral dosing.
ABI-1179 has been evaluated in the Phase 1a portion of a Phase 1a/b study in healthy individuals. A half-life of approximately 4 days was observed across all doses evaluated, supportive of once weekly oral dosing. The Phase 1b portion of the study in individuals with recurrent genital herpes is ongoing.
Recurrent genital herpes
Recurrent genital herpes is a disease caused by herpes simplex virus (HSV) infection that can result in painful genital lesions, serious psychological and social impacts, and an increased risk of acquiring human immunodeficiency virus (HIV). It is estimated that over 4 million individuals in the US and EU5 are affected by recurrent genital herpes. While genital herpes can be caused by either HSV-1 or HSV-2, recurrences are more likely to be experienced by individuals infected by HSV-2. Most individuals with initial symptomatic genital HSV-2 infection have three or more recurrences per year, with painful lesions, lymphadenopathy and urinary problems that can last for 2-3 weeks per recurrence. The current standard of care for recurrent genital herpes is daily chronic suppressive therapy with nucleoside analogs; however, these are only partially effective in preventing recurrences with only 1 in 3 individuals achieving prevention of recurrence. No new drugs have been approved in the United States or Europe to treat genital herpes for more than 25 years.

ABI-6250
ABI-6250 is a small molecule, oral entry inhibitor in development for the treatment of chronic hepatitis delta virus (HDV) infection. ABI-6250 targets NTCP, a human receptor on the surface of liver cells, to block viral infection, which is a clinically validated mechanism. High preclinical potency has been observed with ABI-6250 against multiple HDV strains.
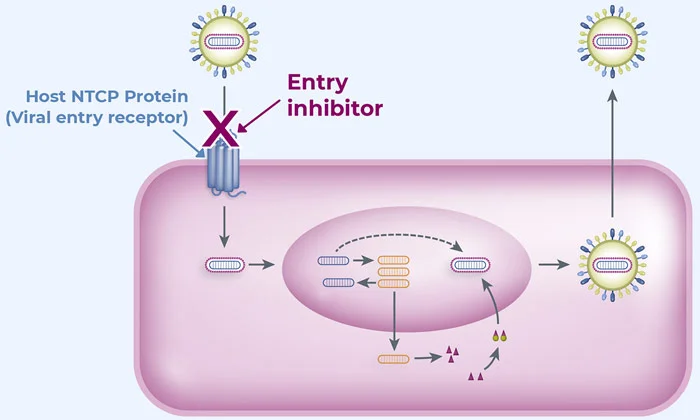
A Phase 1a study with ABI-6250 in healthy individuals demonstrated a 4-day half-life, supportive of daily oral dosing. Further, dose-dependent elevations of a biomarker, total bile acids, were observed, indicating ABI-6250’s engagement with NTCP, the receptor used by HDV to enter hepatocytes.
Chronic hepatitis D virus infection
Chronic hepatitis delta virus (HDV) infection is a serious, life-threatening disease that impacts an estimated 12-72 million individuals worldwide. HDV is a satellite virus that only infects individuals already living with chronic HBV. Coinfection with HDV and HBV increases an individual’s disease burden compared to being infected with HBV alone, with 70% of individuals living with chronic HDV infection progressing to cirrhosis within 10 years. Treatment options for individuals with chronic HDV infection are limited; currently, the only approved treatment is a large molecule entry inhibitor that requires daily injection and cold storage and is only available in Europe.
ABI-4334
ABI-4334 is an orally bioavailable next generation capsid assembly modulator in development for the treatment of chronic hepatitis B virus (HBV) infection. ABI-4334 has been optimized to potently disrupt both viral replication as well as replenishment of new covalently closed circular DNA (cccDNA), the viral reservoir that drives HBV persistence.
In a completed Phase 1b study of ABI-4334 in individuals with chronic HBV infection, ABI-4334 showed a favorable safety and tolerability profile with a pharmacokinetic profile supportive of once-daily oral dosing and potent antiviral activity as measured by viral DNA levels.
Chronic hepatitis B virus infection
Chronic hepatitis B virus (HBV) infection is a debilitating disease of the liver that afflicts approximately 254 million individuals worldwide. HBV infection is the leading cause of chronic liver disease and the need for liver transplantation, with up to 1.1 million deaths due to HBV-related causes per year. The current standard of care for people with chronic HBV infection is life-long suppressive therapy, which reduces but does not eliminate HBV, resulting in very low cure rates.
Research and discovery programs
Assembly Bio has multiple research programs focused on the discovery of additional novel antivirals to target serious viral diseases, including a program focused on discovering a broad spectrum non-nucleoside polymerase inhibitor for the treatment of transplant-associated herpesvirus infections.
More information about our clinical trials can be found on our Clinical Studies page.





